Making COVID-19 vaccines affordable and accessible for everyone
Many COVID-19 vaccines are being developed after the initial publication of the genetic sequence of the virus in January. Some of the clinical trials are currently in phase III, the last phase before becoming available for use. However, this use might not be available for all. There are certain factors that make it more difficult for people who live in rural areas, in a hot climates, and with a higher poverty rate. Tweets by Hugo Slim, specialist in humanitarian aid, inspired us to write an article about this.
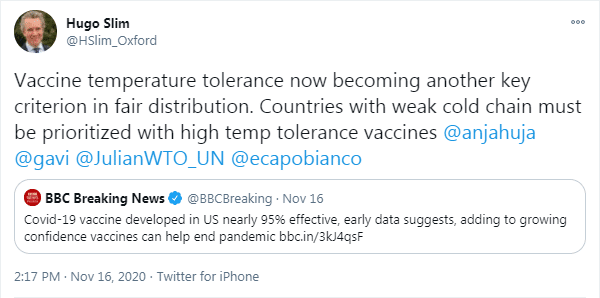
The global fairness of COVID-19 vaccines
The BBC article Slim mentioned in his first tweet, focused on the Moderna COVID-19 vaccine. Next to that, they briefly mention the Pfizer/BioNTech and Sputnik V vaccines. The first tested at an almost 95% effectiveness, the second at approximately 90%, and the third at 92%. The article goes more into detail about how the vaccine works and we highly recommend the read, if you would like to know more about it.
The distribution of the vaccines will take place as soon as possible, but the problem lies in the context in which vaccination campaigns need to take place. While the Pfizer vaccine needs to be stored at a -75ºC temperature to remain stable, the Moderna vaccine ‘only’ needs to be stored at -20ºC and can be handled at room temperature for some time (exact details to be verified) . The reasonable thing to do – as suggested by Slim in his tweet and many humanitarians across the globe – is to make the vaccine that can be stored at the highest temperature the default vaccine for regions with a weak or difficult cold chains. Contexts that might not have the capacity to store a vaccine at -75ºC, whereas other countries or context would. However, this does not seem to be the plan, as of yet.
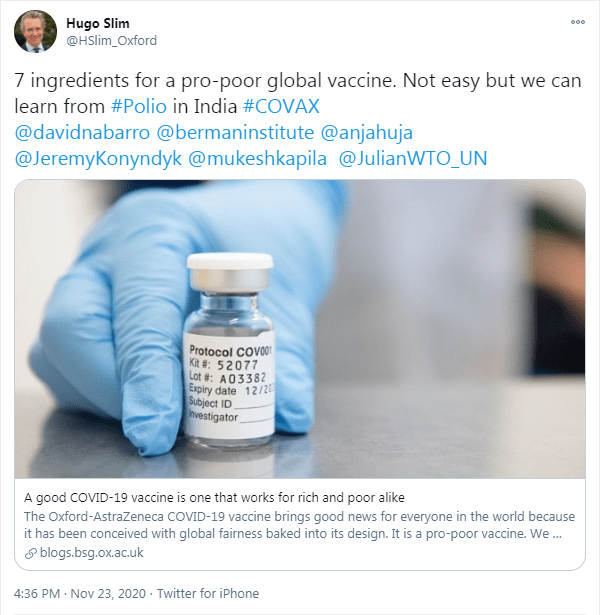
A ‘pro-poor vaccine’
In October, Médecins Sans Frontières (Doctors Without Borders) released a list of four steps that should lead to an affordable and accessible COVID-19 vaccine:
- The research and production of the vaccine should be transparent
- Add conditions to research funding, such as the purpose and availability of the vaccine.
- The distribution of the vaccine should be fair for all.
- Sell the vaccines at the price of production.
The last point relates to the second article Slim mentioned on Twitter. This article calls the Oxford-AstraZeneca COVID-19 vaccine a ‘pro-poor vaccine’ on five of the seven criteria. These criteria are that the vaccine…
☑ tolerates heat, making it usable in regions with a weak cold chain,
☑ can be locally manufactured,
☑ is not too expensive,
☑ protects people of all ages,
☑ integrates easily into the current health system,
☐ prevents the disease as well as transmission and
☐ can be easily delivered, without the need of a health technician of some sort.
The goal is to fulfill every criterion, but this is a strong start for a so-called pro-poor vaccine. The article also mentions other concerns like gaining the public’s trust, vaccination in conflict areas, and overcoming patriarchal control. Once again, we recommend reading the entire article for more information about this.
Responsible vaccine distribution
Shouldn’t the COVID-19 vaccine be distributed in an ethical, accessible and accountable way? What if a vaccine will become available that has the highest percentage of effectiveness, but also the highest storage temperature? Of course, everyone deserves the most effective vaccine, but the higher storage temperature also makes it the best candidate for regions with a weak cold chain. Going further, and when you know which vaccine is to be distributed where, the next step will be how to organise its logistics and the campaign itself, based on the parameters given by the chosen vaccine.
The Solvoz platform can be a catalyst when it comes to sharing expertise and procurement in preparation of the mass vaccination and testing campaigns (among other solutions). Our platform is on the verge of going live and is ready to deliver support to the eradication of COVID-19 and support our responders in the mass vaccination (and testing) campaigns ahead of us. With that, we need your help. Firstly, we need financiers’ help to scale the platform to its largest potential and to enable experts to open-up expertise, in an open-access environment. Solvoz is preparing to launch a project to start building solutions and open up expertise for mass testing campaigns. Think about:
- Cold chain packaging materials and equipment for transportation, storage and distribution of the vaccines and samples;
- Temperature monitoring equipment for vaccines, both on site and remote;
- Logistics equipment and services for vaccination and testing sites, including all the necessary for infection prevention during big gatherings of people;
- Waste management and reverse logistics to safely deal with potentially infectious waste;
- Develop scalable solution packages for testing campaigns.

Secondly, we need the support of suppliers, to add their solutions, products and services to our platform. With the COVID-19 solutions database and the integrated tender workflow, Solvoz creates impact globally both for planning and procurement in COVID-19 responses worldwide, in order to:
- enable transparency and facilitate funding,
- provide open access expert knowledge,
- match needs with solutions,
- gain insight into the worldwide, regional and/or local demand and supply,
- create a networked marketplace, and
- enable stockpiling visibility for future outbreaks or aid responses.
If you are interested in contributing, join our project call, or support in any way, please contact Solvoz for more information.

